Which Statements Describe What Happens in a Redox Reaction
Redox reaction is defined as the reaction in which oxidation and reduction reaction occur simultaneously. B It loses electrons and loses potential energy.
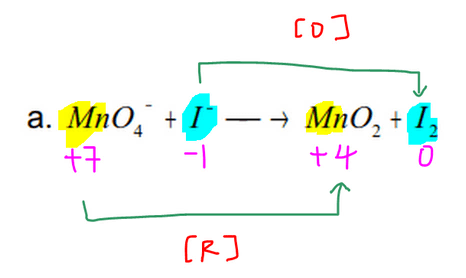
Balance Redox Reaction How To Balance Redox Equation In Acidic Or Alkaline Medium By Maverick Puah The Chemistry Guru Medium
The reaction H2 F2 2HF is an example of a redox reaction.

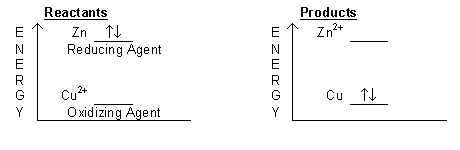
. If Both Assertion And Reason Are True And Reason Is The Correct Explanation Of Assertion. D Zn is the reducing agent and it reduces Cu2. What happens in a Oxidation-Reduction Redox Reaction.
2Mg CO2 2MgO C Which statement describes what happens in this reaction. 3 Which one of these reactions is not an oxidation-reduction reaction. Answer A Carbon is oxidised.
-An atom or molecule loses one or more electrons via reduction-An atom or molecule gains one or more electrons via oxidation-They involve the transfer of hydrogens-They involve the transfer of electrons. The oxidation half-reaction occurs before the reduction half-reaction. 18 Zinc oxide is amphoteric.
You need not work out the oxidation numbers of the other. The reaction H2 F2 2HF is an example of a redox reaction. Which Statements Describe What Happens in a Redox Reaction.
Question 2 2 points Which of the following statements describes what happens to a molecule that functions as the reducing agent electron donor in a redox or oxidation-reduction reaction. It gains electrons and loses potential energy C. Determining whether a reaction is a redox reaction or a non-redox reaction.
Click hereto get an answer to your question The reaction between magnesium and carbon dioxide is shown in the equation. The half reaction H2 2H 2e- is a n. 4 What is the reducing agent in this reaction.
It loses electrons and loses potential energy. In reality two half reactions are occurring. OC it gains electrons and gains potential energy.
17 An example of a redox reaction is shown. 2 Only charge is conserved. In the fourth picture the particle is greatly reduced in size and is contained an irregularly-shaped.
A the electron acceptor is oxidized B the organic substance that loses hydrogen is usually reduced C both decomposition and electron exchange occur D the reaction is uniformly reversible Answer. A It gains electrons and gains potential energy. 2 What is the overall equation for the chemical reaction Zn.
In the first two pictures a cell reaches out and engulfs a particle to create a bubble inside the cell. A It loses electrons and gains potential energy. Electrons are transferred from one reactant to another and the oxidation statesoxidation number of certain atoms are changed.
The reaction between magnesium and carbon dioxide is represented by the following equation. A Zn is the oxidising agent and it oxidises Cu2. Also are all chemical reactions redox.
It gains electrons and gains potential energy. Redox reaction is a type of chemical. Loses electrons and loses potential energy.
It gains electrons and loses potential energy. C Zn is the reducing agent and it oxidises Cu2. 20 What happens in redox reactions.
The electrons are transferred during a redox reaction. Cus 2Ag aq Cu2 aq 2Ags 1 Only mass is conserved. Oxidation reaction is defined as the reaction in which an atom looses its electrons.
Zn Cu2 Zn2 Cu Which statement about the reaction is correct. 2Mg CO2 2MgO C Which statement describes what happens in this reaction. It gains electrons and gains potential energy B.
An oxidation-reduction reaction is any chemical reaction in which the oxidation number of a molecule atom or ion changes by gaining or losing an electron. Thus the correct answers are Option A and Option B. The model illustrates a process by which a substance is taken up by a cell.
Redox reactions are common and vital to some of the basic. B Zn is the oxidising agent and it reduces Cu2. Series of four pictures.
3 Both mass and charge are conserved. An oxidation-reduction redox reaction is a type of chemical reaction that involves a transfer of electrons between two species. The oxidation half-reaction and the reduction half-reaction occur simultaneously.
A If one of the elements shows a change in oxidation number it is sufficient to conclude that the reaction is a redox reaction. Which of the following statements describes what happens to a molecule that functions as the reducing agent electron donor in a redox or oxidation-reduction reaction. Which of the following statements describes what happens to a molecule that functions as the reducing agent electron donor in a redox or oxidation-reduction reaction.
Which of the following statements describes what happens to a molecule that functions as the oxidizing agent electron acceptor in a redox or oxidation-reduction reaction. B It gains electrons and loses potential energy. 1 Which statement best describes what is taking place in this half reaction Fe.
Which statement correctly describes a redox reaction. Which statement is true for all redox reactions. Asked Aug 25 2019 in Biology Microbiology by Brazilian.
In a redox reaction one atom gain electron and another atom looses electron or it can also be said that electrons are transferred from one substance to another substance. Which of the following statements describes what happens to a molecule that functions as the reducing agent electron donor in a redox or oxidation- reduction reaction. In non-redox reactions the oxidation numbers of all elements remain unchanged.
5 Which identifies an oxidation-reduction reaction. 6 In what order are redox reactions. Solved Question 10 Which Of The Following Statements About Redox Reaction Is Correct Navhavc Molc Ahan Orn Ansict Ic Emlts Llght Oxidant Galns Electrons Ad Reduced Chemical State Does Not Change.
In the third picture a small gray cell structure fuses with the bubble. C It gains electrons and loses potential energy. Which statement describes what occurs in the following redox reaction.

Solved Question 13 The Following Redox Reaction Is Spontaneous A5 Written At 259c 3 Fe S 2 Cr3t Aq 3 Fe2t Aq 2 Cr S From The Following Statements Which One Is True Chromium Is

Balancing A Redox Equation In Basic Solution Worked Example Video Khan Academy
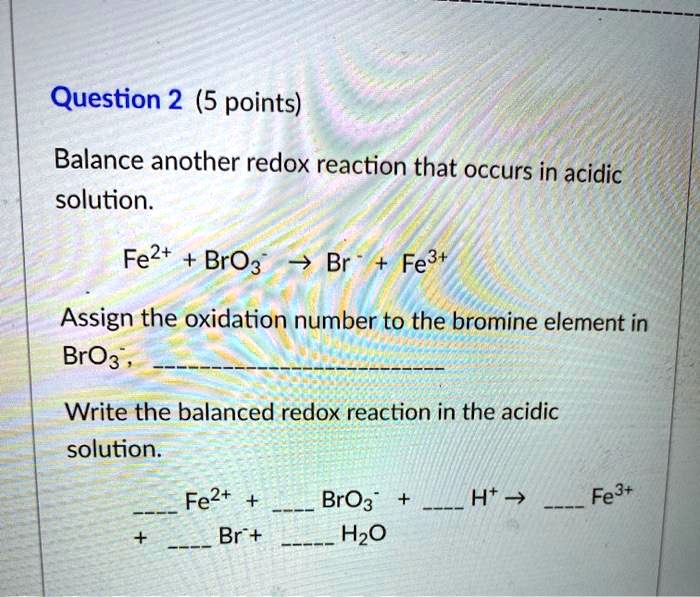
Solved Question 2 5 Points Balance Another Redox Reaction That Occurs In Acidic Solution Fe2 Bro3 Br Fe3 Assign The Oxidation Number To The Bromine Element In Bro3 Write The Balanced Redox Reaction

Spontaneity And Redox Reactions Video Khan Academy

Solved Q16 Classify Each Of The Following Statements As True Or False Oxidation Takes Place At Anode T Er Reduction Takes Place At Cathode T74e Electrolysis Is A Spontaneous Reaction False The Oxidation Number

Electrochemistry Multiple Choice
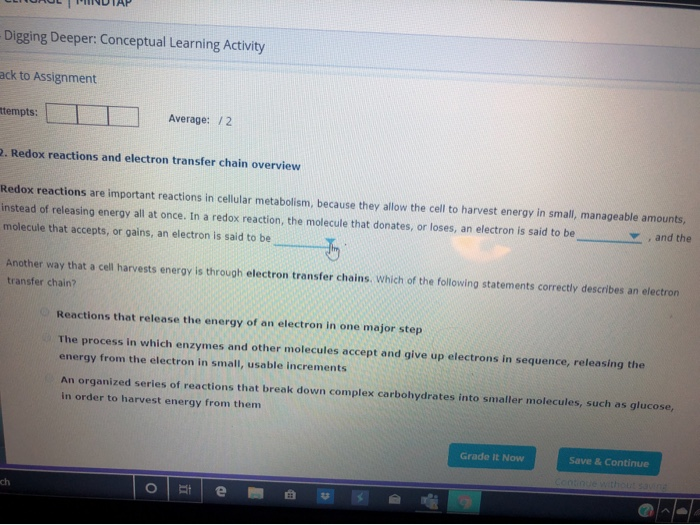
Solved Redox Reaction Are Important Reactions In Cellular Chegg Com

Solved Indicate Whether The Statement Is True Or False If False Change The Identified Word Or Phrase To Make The Statement True 7 Marks 11 The Algebraic Sum Of The Positive And Negative
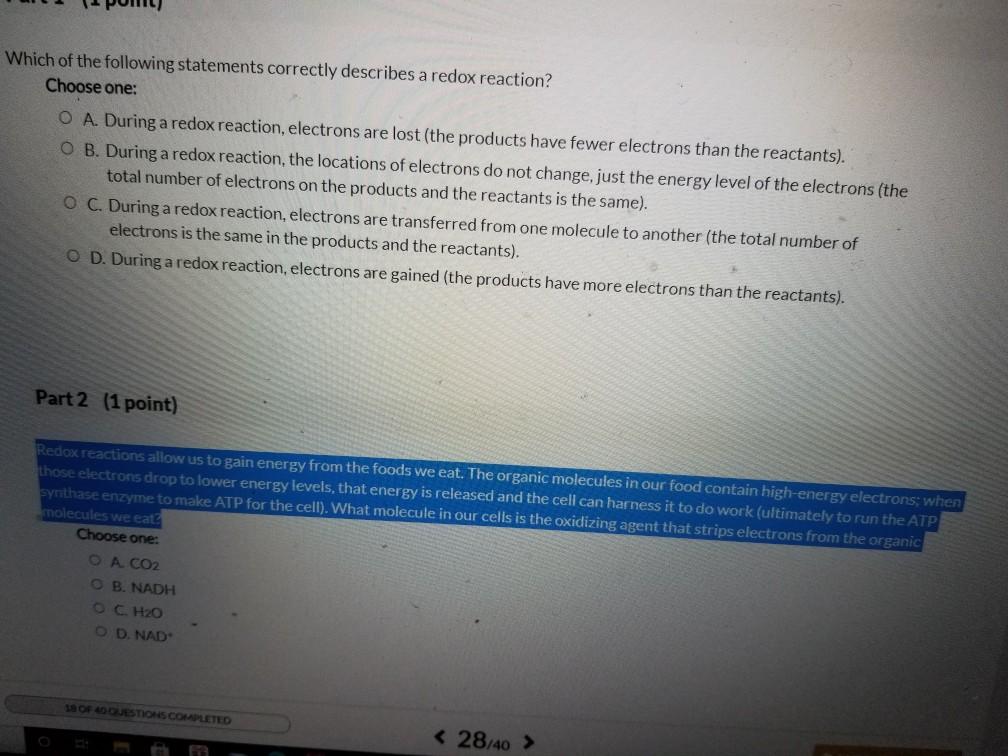
Solved Which Of The Following Statements Correctly Describes Chegg Com

Which Of The Following Reactions Is Not A Redox Reaction Youtube
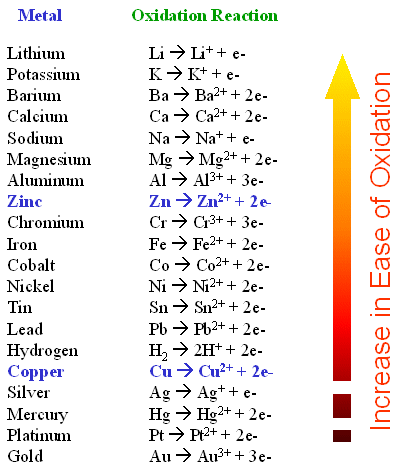
Other Oxidation Reduction Reactions

Redox Reactions Biology For Majors I
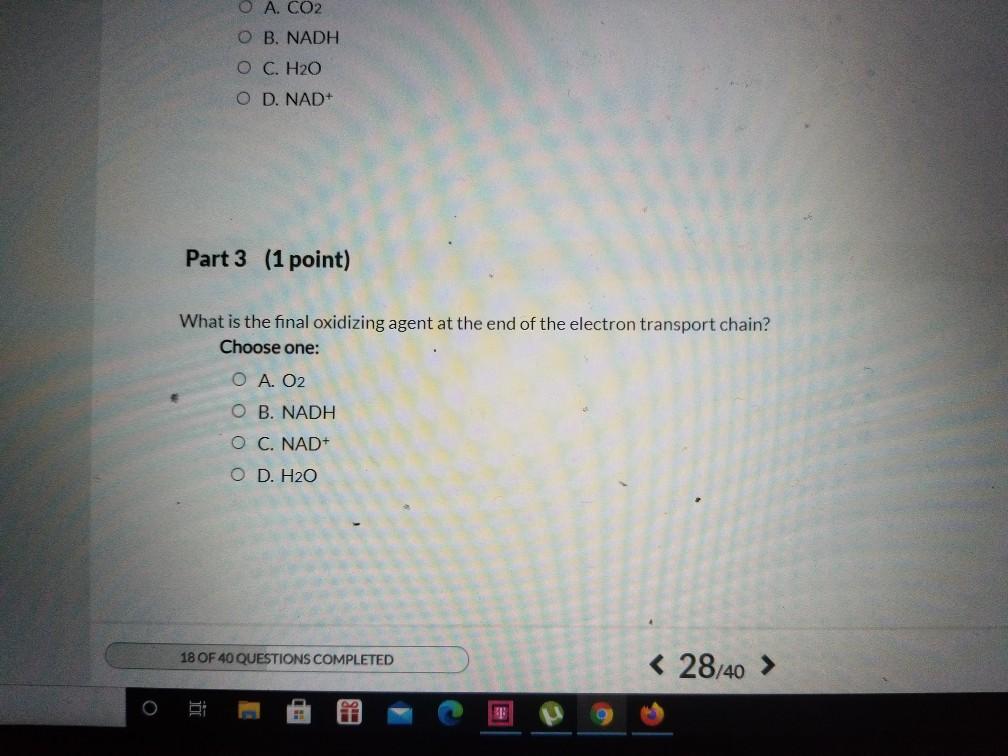
Solved Which Of The Following Statements Correctly Describes Chegg Com


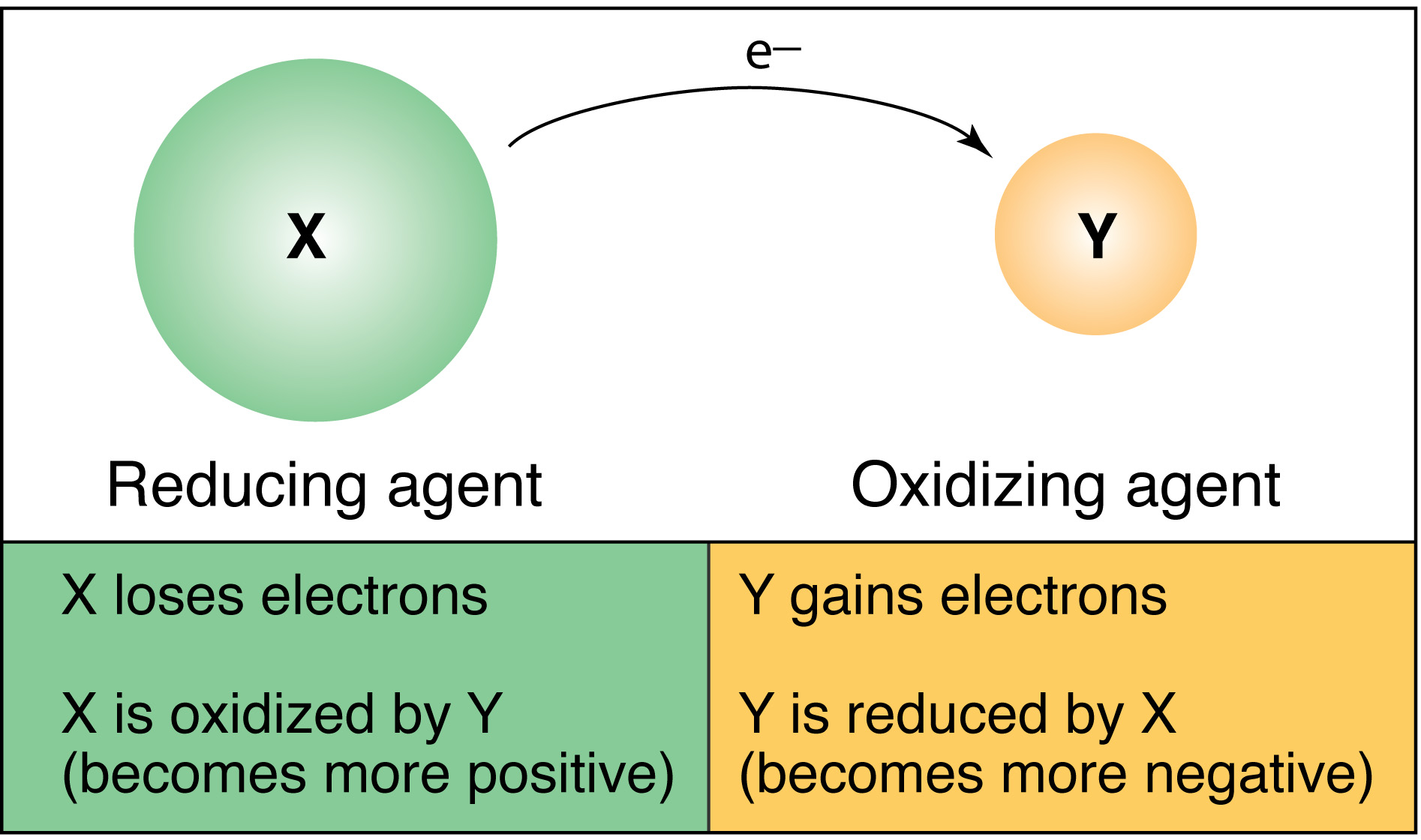
Comments
Post a Comment